Research topics
Team 1: Communication and extracellular transport
Communication and Extracellular Transport
Team 2: NanoBioTherapies
Group 1
Bioproduction of subcellular Biotherapies
Group 2
Advanced physical tools
Group 3
Circulating Nanomarkers for biotherapy
Group 4
now emerging team 3
Team 3 (équipe émergente): Innovative Cancer Treatments
Innovative Cancer Treatments

Team IVETh – national biotherapy-bioproduction integrator dedicated to extracellular vesicles
Extracellular Vesicles : production, engineering and characterization

Team ATIP Avenir: Mucosal viral immune responses
Mucosal Viral Immune Responses (MuVIRep)
Team 1: Communication and Extracellular Transport
Team Leader: Grégory Lavieu (Research Director, INSERM)
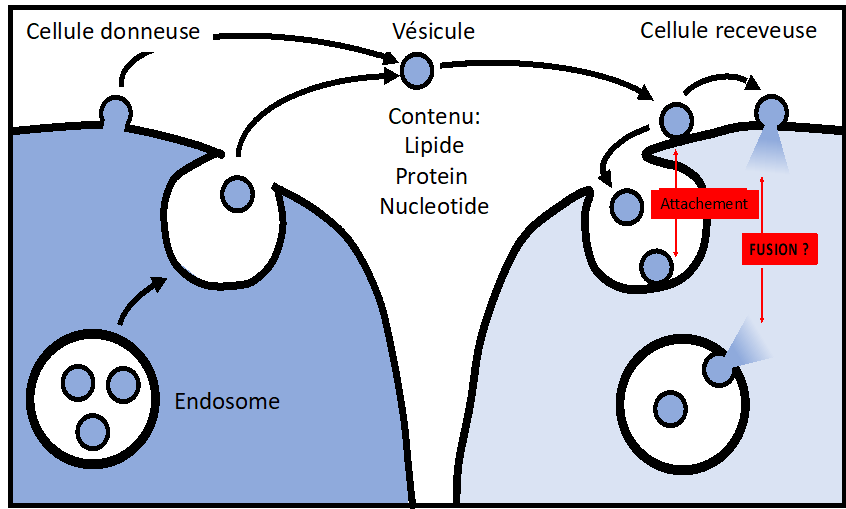
The team focuses on cell-cell communication through membrane transport. We have particular interest for Extracellular Vesicle-mediated transport, Mitochondria intercellular transfer and Virus spreading through cell-cell Fusion.
Research Topic I: EV and Mitochondria Intercellular transport
Our team is dedicated to unraveling the molecular and cellular mechanisms governing extracellular vesicle (EV) delivery, a process broadly involved in physiology and diseases. Over the past five years, we have characterized EV uptake and delivery processes at the cellular level, addressing a major gap in the field. Building on this knowledge, we have successfully engineered EV-based vectors for targeted therapeutic delivery. Currently, we are leveraging Cas9 genetic screening in combination with our unique cellular assays to identify and characterize the core machinery involved in EV uptake and delivery, with a particular focus on membrane docking and fusion.
Recent evidences suggest that mitochondria may also travel between cells. Using our established methodologies, we aim to characterize this emerging intercellular trafficking route. Our goal is to dissect the molecular and cellular mechanisms underlying mitochondrial transport, just as we have done for EVs. These insights will directly contribute to optimizing mitochondrial transplantation, a promising avenue for translational medicine.
Team Members :
Julia Dancourt, PhD (Assistant Professor, Universté Paris Cité)
Julia is leading the genome-wide screenings effort to identify key actors of EV-delivery.
Sheryl Bui, PhD, (Postdoctoral fellow and MD student)
Sheryl aims at developing EV-mimetics for targeted therapeutics delivery
Pierre Tapie, PhD, (Postdoc)
Pierre is developing in vitro imaging assay to demonstrate EV fusion in realtime.
Shaghayegh Askarian, PhD, (Postdoc).
Shaya is characterizing extracellular mitochondria delivery at both cellular and molecular level
Ronald Saraswat, PhD, (Postdoc)
Ronald is developing new assay to perform genome wide genetic screening toward EV-content delivery.
Maud Chevé, Engineer,
Maud provides expertise and support in cell biological aspects of the EV-based projects. She is also involved in the production of bioengineered EV for therapeutical applications.
Yacine Lahmer, (Master student2)
Yacine is testing and characterizing protein candidates that act as l negative or positive regulator of EV uptake.
Alumni
Emeline Bonsergent (PhD student), Zahra El Amir Dache (postdoc), Jihad Karam (postdoc)
Selected Relevant Publications:
- Tognoli ML, Dancourt J, Bonsergent E, Palmulli R, de Jong OG, Van Niel G, Rubinstein E, Vader P, Lavieu G. Lack of involvement of CD63 and CD9 tetraspanins in the extracellular vesicle content delivery process. Commun Biol. 2023 May 17;6(1):532. doi: 10.1038/s42003-023-04911-1. PMID: 37198427; PMCID: PMC10192366.
- Bui S, Dancourt J, Lavieu G. Virus-Free Method to Control and Enhance Extracellular Vesicle Cargo Loading and Delivery. ACS Appl Bio Mater. 2023 Mar 20;6(3):1081-1091. doi: 10.1021/acsabm.2c00955. Epub 2023 Feb 13. PMID: 36781171; PMCID: PMC10031566.
- Dancourt, J., Piovesana, E. & Lavieu, G. Efficient cell death mediated by bioengineered killer extracellular vesicles. Sci Rep 13, 1086 (2023)
- Bonsergent E, Grisard E, Buchrieser J, Schwartz O, Théry C, Lavieu G. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat Commun. 2021 Mar 25;12(1):1864. doi: 10.1038/s41467-021-22126-y. PMID: 33767144; PMCID: PMC7994380
- Bonsergent E, Lavieu G. Content release of extracellular vesicles in a cell-free extract. FEBS Lett. 2019 doi: 10.1002/1873-3468.13472.
- Spotlight on early-career researchers: an interview with Gregory Lavieu. Commun Biol. 2018. doi: 10.1038/s42003-018-0144-1.
Patents:
- Extracellular vesicles functionalized with an erv syncitin and uses thereof for cargo delivery wo pct/ep2023/070159
- Extracellular vesicles functionalized with a tethering system for cargo delivery ep23306248.8
Research Topic II: HIV-1 cell-to-cell spreading in myeloid cells
Myeloid cells, including macrophages but also dendritic cells (DCs) and osteoclasts (OCs) are emerging as import target cells of HIV-1 playing critical roles in the pathogenesis of AIDS. Because these myeloid cells are poorly infected by cell-free viruses, cell-to-cell transfer of HIV-1 may represent the dominant mode of virus dissemination in vivo and may allow for productive infection of these cell types. Virus cell-to-cell spread facilitates rapid viral dissemination between the target cells but may also promote immune evasion and contribute to pathogenesis.
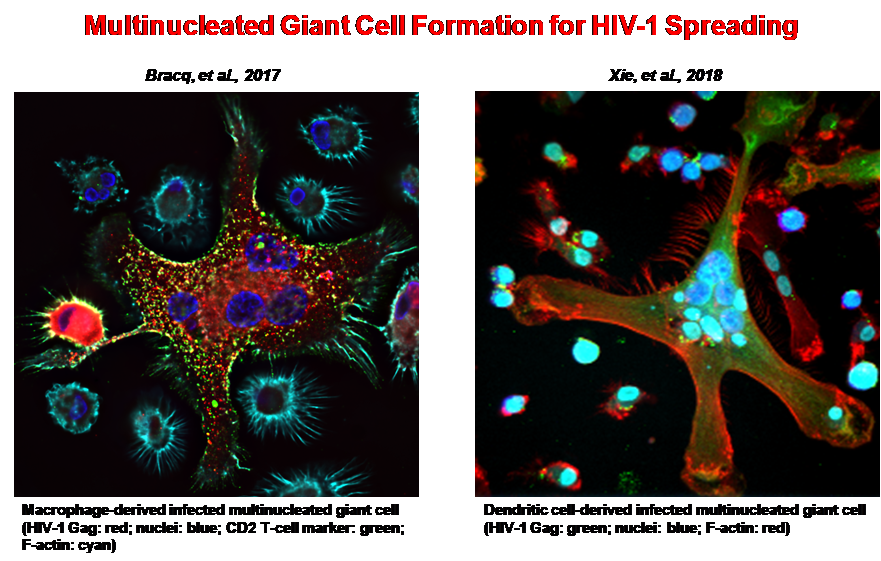
The general goal of our project is to characterize the cellular and virological mechanisms involved in HIV-1 cell-to-cell transfer and dissemination in macrophages and other myeloid cells, as well as the consequences of this virus transfer on macrophages functions. We have already demonstrated that HIV-1 uses a very efficient mechanism for virus cell-to-cell transfer from infected CD4 T cells and dissemination in macrophages, DCs and OCs by a two-step cell-cell fusion process leading to the formation of highly virus-productive multinucleated giant cells. We are now characterizing the cellular factors and signaling pathways modulating virus cell-to-cell dissemination from infected T cells by cell-cell fusion with macrophages.
Team Members:
Serge Benichou, PhD (CNRS Research Director)
Serge’s research group aims at characterizing HIV spreading through cell-cell fusion.
Sen Yan, (PhD student)
Sen is analyzing the role of the host cell restriction factor BST-2 for HIV-1 cell-to cell spreading in macrophages.
Agathe Durringer, (PhD student)
Agathe is developing genetic screenings for identification of new cellular factors involved in HIV-1 cell-to cell spreading in macrophages.
Selected Relevant Publications (since 2017):
- Woottum, M., Yan, S., Sayettat, S., Grimberg, S., Bekaddour, N., Cathelin, D., Herbeuval J.-P. and Benichou, S. (2024) Macrophages: key cellular players in HIV-1 infection and pathogenesis. Viruses, 16, 288.
- Harms*, M., Smith*, N., Han, M., Groß, R., von Maltitz, P., Rodriguez-Alfonso, A., Sayettat, S., Bekaddour, N., Vanshylla, K., Wiese, S., Ständker, L., Klein, F., Lagane, B., Benichou, S., Sanchez-Garcia, E., Herbeuval, J.-P. and Münch, J. (2023) Spermine and spermidine bind CXCR4 and inhibit CXCR4- but not CCR5-tropic HIV-1 infection. Science Advances, 9, eadf8251. (*equal contribution).
- Mascarau, R., Woottum, M., Fromont, L., Cantaloube-Ferrieu, V., Vahlas, Z., Bertrand, F., Beunon, T., Métais, A., Jabrane-Ferrat, Nabila, Gallois, Y., Guibert, N., Favre, G., Maridonneau-Parini, I., Poincloux, R., Lagane, B., Benichou, S., Raynaud-Messina, B. and Vérollet C. (2023) Productive HIV-1 infection of tissue macrophages by fusion with infected CD4+ T cells. J. Cell Biol., 222(5): e202205103.
- Han, M., Cantaloube-Ferrieu, V., Xie, M., Armani-Tourret, M., Woottum, M., Pagès, J.-C., Colin, P., Lagane, B. and Benichou,S. (2022) HIV-1 cell-to-cell spread overcomes the virus entry block of non-macrophage-tropic strains in macrophages. PLoS Pathogens, 18(5): e1010335.
- Han, M., Woottum, M., Mascarau, R., Vahlas, Z., Verollet, C. and Benichou, S. (2022) Mechanisms of HIV-1 cell-tocell transfer in myeloid cells. J. Leuk. Biol., 1-11.
- Xie, M., Leroy, H., Mascarau, R., Woottum, M., Dupont, M., CiccSchmitt, A., Raynaud-Messina, B., Vérollet, C., Bouchet, J., Bracq, L., and Benichou, S. (2019) Cell-to-cell fusion for HIV1 spreading and SAMHD1-independent productive infection of myeloid target cells. mBio 10(6), e02457-19.
- Bracq, L., Xie, M., Benichou, S*., and Bouchet, J*. (2018) Mechanisms for cell-to-cell transmission of HIV-1. Front. Immunol., 9, 260. (*Corresponding authors)
- Raynaud-Messina, B., Bracq, L., Dupont, M., Souriant, S., Usmani, S. Proag, A., Pingris, K., Soldan, V., Thibault, C., Capilla, F., Al Saati, T., Gennero, I., Jurdic, P., Jolicoeur, P., Daviginon, J.-L., Mempel, T., Benichou, S., Maridonneau-Parini, I. and Verollet, C. (2018) The bone degradation machinery of osteoclasts: a novel HIV-1 target that contributes to bone loss. Proc. Natl. Acad. Sci. USA, 115(11), E2556-E2565.
- Bracq, L., Xie, M., Lambelé, M., Vu, L.-T., Matz, J., Schmitt, A., Delon, J., Zhou, P., Randriamampita, C., Bouchet, J., and Benichou, S. (2017). T cell-macrophage fusion triggers multinucleated giant cell formation for HIV-1 spreading. J. Virol., 91, e01237-17.
Team 2: NanoBioTherapies
Group 1: Bioproduction of subcellular Biotherapies
Bioproduction and engineering of extracellular vesicles by turbulence for digestive fistula therapy in thermoreversible hydrogels
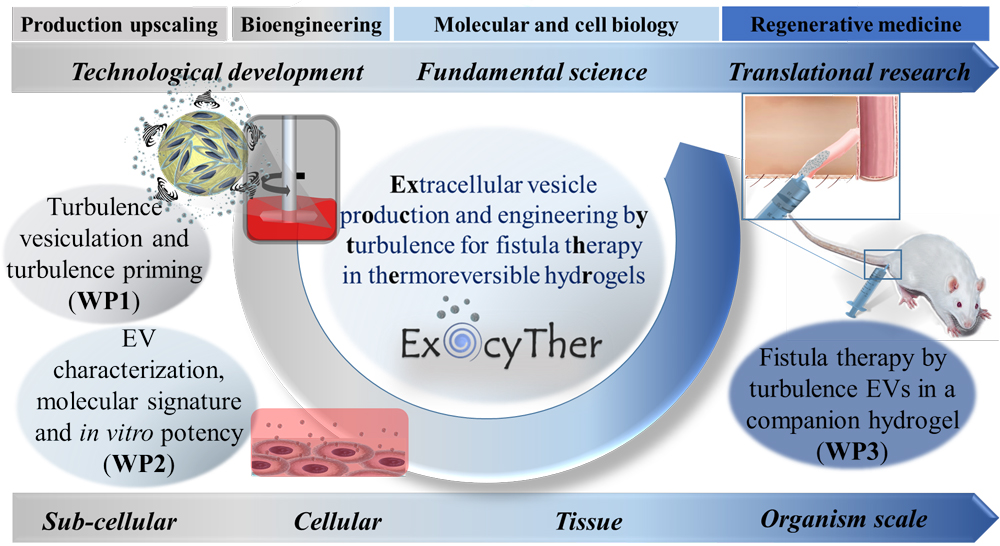
We are exploring digestive fistula therapy using extracellular vesicles (EVs) derived from stromal cells (SCs), capable of reproducing the therapeutic effects of their parent cells. This approach aims to reduce the risks associated with uncontrolled replication, differentiation, and vascular occlusion, while offering advantages in terms of off-the-shelf availability, storage, and shelf life. However, the clinical translation of SC EV therapy remains limited by challenges related to their production, engineering, and administration.
As part of the ExocyTher project, funded by the European Research Council (ERC) with a total budget of 1.5 million euros, we aim to develop SC EV therapy along two main lines:
- Standardized, scalable, and high-yield EV production: we propose the concept of turbulence-induced vesiculation, which stimulates the release of EVs from SCs by applying controlled turbulent shear integrated into large-scale bioreactor-based cell culture.
- Optimized delivery: we propose the administration of EVs in a synergistic occlusive companion gel (injectable below 20°C and gelling at body temperature) with the goal of filling the entire fistula tract and retaining EVs at the target site.
ExocyTher addresses the treatment of fistulas, a major health burden associated with Crohn’s disease, or secondary to surgery, cancer therapy, or trauma. These conditions affect millions of people in Europe and are associated with a high rate of morbidity.
The all-in-one strategy, combining turbulence-induced vesiculation and EV priming, is expected to yield high quantities of SCs-derived EVs with enhanced immunomodulatory properties, particularly for more efficient treatment of inflammatory Crohn’s disease-related fistulas.
Building on the use of a medical-grade gel already authorized in Europe (used off-label as an occlusive EV vehicle), and taking into account key regulatory considerations, ExocyTher aims to lay the groundwork for the first clinical trial of EV therapy for fistulas using SCs.
The concepts developed in ExocyTher may also be extended to other EV-producing cell types or therapeutic applications, with significant scientific and technological impact anticipated.
Collaborators
Gabriel Rahmi, Hôpital Européen Georges-Pompidou, Paris Centre de Recherche Cardiovasculaire (PARCC) – INSERM – Université Paris Cité, Paris
Noëlle Mathieu, Institut de Radioprotection et de Sûreté Nucléaire (IRSN), Fontenay-aux-Roses
https://cordis.europa.eu/project/id/852791
Extracellular vesicles from genetically modified stromal cells for immunomodulatory enzyme replacement biotherapy
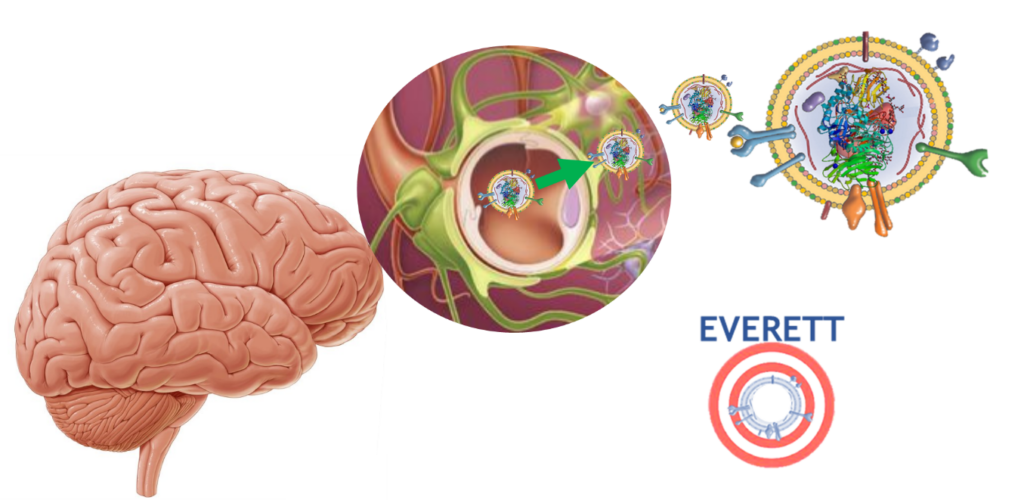
We are studying extracellular vesicles (EVs) for the delivery of therapeutic proteins to the central nervous system (CNS) in the context of neurodegenerative diseases. EVs derived from stromal cells (SC-EVs) have the ability to cross the blood-brain barrier, target the brain, and reduce neuroinflammation thanks to their immunomodulatory effects. These SC-EVs can be loaded with a recombinant protein whose overexpression is induced by the genetic modification of their parent cells. These nanovesicles can thus carry the therapeutic protein, protect it from the immune system, and deliver it to target cells. This is the aim of the EVERETT project, with a total budget of €1.4 million, funded by the Programme d’investissements d’avenir (PIA), as part of the Grand Défi Biomédicaments.
The overall objective of the EVERETT project is to optimize an integrated high-throughput production process for SC-EVs that are both safe and effective, and to initiate an affordable industrial transfer of this new immunomodulatory biotherapy targeting the brain and capable of delivering therapeutic proteins.
Our hypothesis is that SC-EVs are ideal nanocarriers for treating both protein deficiencies and inflammation in the brain, particularly in the context of lysosomal diseases that affect the CNS and are currently incurable.
With the ambition of moving these biotherapies toward clinical application, our public/private consortium aims to optimize the entire SC-EV manufacturing process.
Partners
Corinne Sagné, Saints-Pères Paris Institute for the Neurosciences (SPPIN), Université Paris Cité, CNRS UMR 8003, Paris 75006, France.
Ibane Abasolo, Vall d’Hebron Institute of Research (VHIR), Universitat Autònoma de
Barcelona (UAB), Barcelone
Jean-Roch Fabreguettes, Centre MEARY de Thérapie Cellulaire et Génique de L’Assistance Publique-Hôpitaux de Paris (AP-HP), Paris
Jeanne Volatron, Everzom, Paris
[Investissements d’avenir] Le projet EVERETT lauréat de l’AAP « Nouveaux systèmes d’expressions »
A matrix-based therapy to optimize extracellular vesicle for protecting and repairing the ischemic brain
We are studying regenerative therapy based on the use of stromal cells (SCs) as producer cells for a biotherapy aimed at treating stroke (ischemic cerebrovascular accidents). The beneficial effects of SCs appear to be mainly linked to the secretion of cellular factors and/or extracellular vesicles (EVs). That’s why, we will focus on EV-based biotherapies. Heparan sulfates present on the surface of both producer and recipient cells, as well as on the EVs themselves, may play a critical role in the communication mediated by these vesicles.
As part of the MAESTROVE project, funded by the ANR with a total budget of €599,874, we aim to determine whether combining EVs derived from human SCs with a heparan sulfate-mimicking agent enhances their effects on tissue regeneration and functional recovery after stroke treatment. This could open a new and fast-track avenue for the industrial development of a stroke therapy.
Partners:
Myriam BERNAUDIN, Imagerie et stratégies thérapeutiques des pathologies cérébrales et tumorales (ISTCT), Caen
Franck Chiappini, Société OTR3 Organes Tissus Régénération Réparation Remplacement, Paris
Dulce Papy-Garcia, GLY-CRRET Laboratoire de recherche sur la croissance cellulaire, la réparation et la régénération tissulaires, Créteil
Extracellular Vesicles and Intervertebral Disc Therapy
We are studying extracellular vesicles (EVs) as an alternative to cell-based therapies for the intervertebral disc (IVD). Low back pain, which affects over 80% of adults during their lifetime and has significant socioeconomic consequences, is often associated with IVD damage. An IVD consists of a gelatinous nucleus pulposus (NP) surrounded by a dense annulus fibrosus. IVDD is characterized by the loss of NP cells, dehydration of the NP extracellular matrix, increased expression of matrix metalloproteinases and inflammatory factors, leading to a loss of biomechanical properties, resulting in pain and disability.
Current treatments, medication for early-stage degeneration and surgery for more advanced cases, aim only to control pain and do not address the underlying degenerative processes. Stromal cell (SC)-based therapies could help reverse the degenerative process and restore biomechanical function. While promising results have been obtained with SC from bone marrow or adipose tissue injected into degenerated discs, the underlying therapeutic mechanisms are not yet fully understood.
The regenerative potential of SCs after injection may depend on their ability to secrete bioactive factors, either directly or through the release of EVs, that locally interact with NP cells. While this paracrine effect of SCs has been demonstrated in inflammation-associated degenerative diseases, including osteoarthritis, the use of SC-derived EVs for IVD regeneration is still in its early stages.
As part of the EXCELLDISC project, funded by the ANR with a total budget of €678,240, we hypothesize that SC-derived EVs could serve as an alternative to cell therapies, with certain strategic advantages such as the absence of uncontrolled differentiation and the possibility of long-term storage. Furthermore, we hypothesize that EVs could be produced in response to specific disc-like microenvironments (hypoxia, high osmolarity, acidic pH, nucleus pulposus differentiation medium) that mimic the conditions of a degenerated disc, and that relevant recipient cells could internalize SC-derived EVs both in vitro and ex vivo. Finally, we propose that SC-derived EVs could help slow, halt, or reverse degeneration in an established and clinically relevant ovine model of degenerative disc disease.
Partner:
Catherine Le Visage, Jérôme Guicheux et équipe RMES, (Regenerative Medicine and Skeleton), Nantes Université
https://anr.fr/Projet-ANR-19-CE18-0020
Bioproduction and engineering of extracellular vesicles from Gram-positive bacteria for anti-inflammatory therapeutic purposes
We are studying a biotherapy based on extracellular vesicles (EVs) derived from therapeutic Gram-positive bacteria as an alternative to live probiotics. Large-scale, high-yield EV production in bioreactors will be developed using Gram-positive bacteria with known anti-inflammatory activity, such as Propionibacterium freudenreichii (an industrial strain) and Faecalibacterium prausnitzii (a commensal strain from the gut microbiota). EVs will be characterized both biophysically and molecularly (proteins, lipids, sugars, nucleic acids, metabolites).
This work is part of the BRACTER-EV-BOOSTER project, funded by France 2030, with a total budget of €3.5 million. The project also includes engineering of EV content to enhance their intrinsic immunomodulatory activity. The anti-inflammatory and neuromodulatory properties of EVs from a single species or from a pool of EVs derived from individually cultured species will be tested:
- in vitro in cellular models, and
- in vivo in preclinical models of acute inflammation (Inflammatory Bowel Disease – IBD),
- low-grade inflammation (Irritable Bowel Syndrome – IBS) associated with cognitive-emotional and behavioral disorders, and
- neurodegenerative disease (Alzheimer’s model).
This study aims to address several challenges:
- Biotechnological challenge: vesiculation hindered by the bacterial cell wall
- Therapeutic challenge: matching and adapting the biotherapy to three different therapeutic indications
- Safety challenge: reducing infectious risks
- Pharmaceutical challenge: mastering quality attributes—quantity, identity, purity, and biological activity
- Reproducibility challenge: ensuring batch-to-batch consistency, stability, and storage conditions.
Collaborators:
| Jean-Marc CHATEL – UMR 1319 Agro Paris Tech – INRAE – Université Paris-Saclay Institut de Microbiologie de l’Alimentation au service de la Santé (MICALIS) |
| Michel NEUNLIST – UMR 1235 Inserm – Nantes Université Système nerveux entérique dans les troubles de l’intestin et du cerveau (TENS) |
| Laurent BENEY – UMR Procédés Alimentaires et Microbiologiques (PAM) Institut d’Agronomie de Dijon – Université de Bourgogne Université de Bourgogne Franche-Comté |
| Clotilde POLICAR – UMR 7203 CNRS – ENS – Université Paris Sciences et Lettre – Sorbonne Université Laboratoire des BioMolécules (LBM) |
| Frédéric CARVALHO – UMR 1107 Inserm – Université Clermont Auvergne Douleur Biophysique et Neurosensorielle (NeuroDol) |
https://pepr-biotherapies.fr/2023/12/08/bacter-ev-booster/
Localized delivery of therapeutic RNA via functionalized hybrid vesicles for musculoskeletal regeneration
We are studying extracellular vesicles (EVs) as a next-generation nanocarrier system for the delivery of therapeutic RNAs in regenerative medicine. Therapeutic RNAs, including microRNAs and messenger RNAs (mRNAs), hold great promise for tissue regeneration. However, their clinical use faces major challenges in regenerative medicine, particularly regarding the control of immune responses.
The CARN project aims to develop biopharmaceuticals that use EVs as biologically active systems for the delivery of therapeutic RNAs to regenerate skeletal tissue. EVs isolated from stromal cells (SCs) mimic the therapeutic effects of their parent cells and are of great therapeutic interest in regenerative medicine. Naturally circulating in the body to support intercellular communication, these vesicles carry proteins, lipids, and nucleic acids, especially miRNAs, responsible for their biological effects.
EVs are also being developed as targeted delivery systems due to:
- their ability to transport a therapeutic “cargo” specifically to target tissues;
- their exceptional capacity to escape endosomes and release RNA directly into the cytosol, where it becomes active.
Currently (as recognized by the 2023 Nobel Prize in Medicine), synthetic lipid vesicles, such as liposomes or lipid nanoparticles, are used to deliver RNA (e.g., for COVID-19 vaccines). While these synthetic nanoformulations are effective for vaccines because they induce a strong immune response, they must be modified for other therapeutic strategies, particularly regenerative medicine, where inflammation must be tightly regulated.
In this context, combining synthetic nanoparticles with stem cell-derived EVs into hybrid vesicles offers a promising strategy to merge the immunomodulatory and regenerative properties of EVs with the efficient RNA delivery capacity of synthetic carriers. These hybrid EVs can also be functionalized with recognition elements to specifically target cells in degenerated tissues.
This project builds on the complementary, multidisciplinary expertise of the CARN consortium, funded by France 2030 with a total budget of €3.6 million. We will propose innovative, scalable, and commercially viable methods for the (bio)production of therapeutic RNAs, the bioproduction of EVs from stem cells, and their hybridization to create the next generation of RNA nanocarriers for regenerative applications. Various targeting motifs will also be explored.
The project team proposes that hybrid EVs, combining the targeting and tissue delivery potential of biogenic EVs with the high RNA payload of lipid nanoparticles, could offer a real added value for therapeutic RNA delivery. In the CARN project, both microRNAs (targeting inflammation, apoptosis, senescence) and mRNAs (coding for biological factors such as transcription factors and growth factors involved in skeletal regeneration and SC immunosuppression) will be formulated and encapsulated in innovative functionalized hybrid EVs.
By developing hybrid EVs decorated with targeting elements and loaded with therapeutic RNAs, CARN will open new therapeutic windows for treating skeletal diseases, improving patient care, and contributing to innovation and French sovereignty in the bioproduction of advanced therapies.
Collaborators:
|
Jérôme GUICHEUX – UMRS 1229 |
|
Chantal PICHON – US55 ART-ARNm |
|
Elias FATTAL – UMR 8612 |
|
Marie MORILLE – UMR 5253 |
|
Danièle NOËL – UMR 1183
https://pepr-biotherapies.fr/2023/12/08/carn/ |
From the production of extracellular vesicles isolated from iPSC-derived stromal cells to clinical application
We are studying extracellular vesicles (EVs) isolated from stromal cells (SCs) as biopharmaceuticals capable of reproducing the main therapeutic effects of their parent cells, without the risks of proliferation or differentiation after administration. The therapeutic potential of EVs has been demonstrated in numerous preclinical models and across a wide range of therapeutic applications, particularly in inflammatory, acute, or degenerative diseases. One key example is osteoarthritis, a degenerative disease for which there is currently no curative treatment, affecting a growing number of patients and imposing a significant burden on public healthcare systems.
Although EVs are promising therapeutic tools, one of the major limitations to their use lies in the lack of batch-to-batch reproducibility. This heterogeneity may stem from donor variability, cell expansion protocols, and EV isolation and characterization techniques. Another key limitation to clinical use relates to the volume of EVs required, which calls for large-scale, clinically compatible manufacturing processes.
As part of the STROMAEV project, funded by France 2030 with a total budget of €3.69 million, our ambition is to address these production constraints while generating EVs with strong therapeutic efficacy—allowing for the transfer of a scalable, clinically compatible production process to the biomanufacturing industry.
We propose to produce EVs from SCs derived from universal induced pluripotent stem cells (iPSCs), which are not recognized by the recipient’s immune system and offer a virtually unlimited source of cells with a more stable phenotype and function. In the STROMAEV project, SCs generated from iPSCs will be activated using physical, chemical, or molecular methods to produce “enhanced” EVs. These EVs will be evaluated functionally through in vitro and in vivo tests, including in models of induced osteoarthritis.
A large-scale and clinically compatible production process will be developed, and key quality control attributes will be defined to enable standardization of the process for industrial use.
By establishing this production platform, STROMAEV aims to accelerate the industrial-scale biomanufacturing of therapeutic EVs and their transfer to the clinic for a first-in-human osteoarthritis clinical trial as a proof of concept.
Partners:
|
Danièle NOËL – UMR 1183 |
|
Vincent AGACHE – LETI |
|
Sébastien BANZET – UMR-MD 1197 |
https://pepr-biotherapies.fr/2023/12/08/stromaev/
Group 2: Advanced Physical Tools
The Advanced Physical Tools team is a sub-group within the Nanobiotherapies team of the NABI laboratory. We are physicists dedicated to working at the interface of biology and medicine, employing cutting-edge physical techniques to address key biological and medical questions. Our work spans different scales, ranging from isolated proteins to larger complexes such as liposomes and extracellular vesicles, and extends to multicellular systems like organoids and entire organisms such as embryos, while also focusing on single-cell studies.
Permanent people: Stéphanie Mangenot, Vincent Fleury, Anne Beaudot, Hugo Salmon, Kelly Aubertin, Jean-Marc Di Meglio.
Research Focus
Our research spans multiple interdisciplinary topics, including:
- Cryopreservation Studies: We investigate cryopreservation techniques (slow cooling and vitrification) to enhance the long-term preservation of biological samples. This includes studying the impact of cryoprotective agents, cooling rates and thawing processes on the structural and functional integrity of cells, tissues and extracellular vesicles. Our work aims to improve the viability and functionality of cryopreserved samples, which has significant implications for the development of tissue/cell banks, medically assisted reproduction, rare diseases treatments and facilitates research in regenerative therapy.
- Intracellular and Extracellular Transport: Our research explores the mechanisms of molecular and particle transport within cells and across cellular boundaries. This includes understanding the dynamics of vesicle trafficking, the behavior of nanoparticles in the cellular microenvironment, and the transport of therapeutic agents. These studies are critical for advanc drug delivery systems and understanding cellular communication.
- Cell Membrane Mechanics and properties: We delve into the mechanical properties of cellular membranes, such as their stiffness, elasticity, and deformability. By studying how membranes are deformed and interact in the presence of various proteins, we gain insights into fundamental biological processes such as endocytosis, exocytosis, and cytokinesis. Additionally, we investigate the mechanical behavior of diseased cells, including cancer cells and cells affected by genetic disorders, to uncover potential diagnostic or therapeutic avenues.
- Embryology and Regenerative Medicine: Our team investigates early developmental biology to understand the physical and biochemical cues that govern cellular differentiation and tissue formation. This knowledge is applied to regenerative medicine, aiming to develop innovative therapies for tissue repair and organ regeneration. Our studies include the development of biomimetic systems to replicate embryonic environments.
- Development of frugal instrumentation: we build in the lab, from scratch, diverse experimental set-ups, for studying limb growth in embryos under pressure pulses, for monitoring the activity of mice, of hen braiding, for measuring the elasticity of culture gels, to quote a few examples. Theses set-ups are prototypes of innovative methods for health improvement or new integrated systems to address animal behavior.
Advanced Techniques
We utilize state-of-the-art technologies, not merely as end-users but as developers, continually pushing the boundaries of these methods to expand their applications and capabilities. Key techniques include:
- Atomic Force Microscopy (AFM): We use AFM to probe the mechanical properties of biological samples at the nanoscale. This technique allows us to measure stiffness, adhesion forces, and deformation behavior, providing valuable insights into the physical state of cells, membranes, and other biomaterials. It also allows imaging of sample surfaces.
- Microfluidics: Our team develops microfluidic systems to create controlled environments that mimic physiological conditions. These include organ-on-chip devices for studying tissue responses to stimuli and neuron growth models to investigate neural development and connectivity. Microfluidics enables high precision in studying cellular behavior under dynamic conditions.
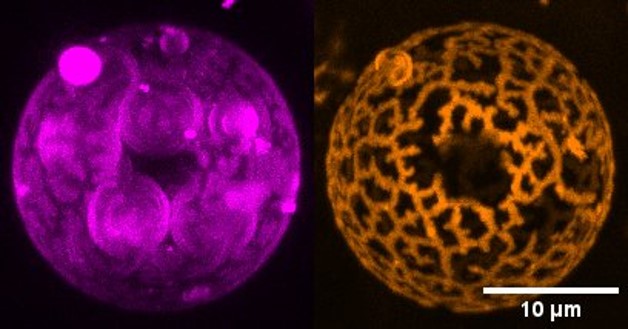
- Super-Resolution Microscopy: By breaking the diffraction limit of conventional light microscopy, we achieve nanoscale imaging of biological structures. This technique is instrumental in visualizing molecular interactions, tracking dynamic processes in live cells, and mapping the architecture of complex biological systems with unparalleled detail.
- Raman microscopy and optical tweezers: we work at the bulk and the nanoscopic level to characterize the global molecular fingerprint of biological samples. For bulk, we use a commercial Raman imaging system giving access to virtual cartography of various biological samples (extracellular vesicles, biofluids, cells, etc.). At the nanoscopic level, we work with a “lab-made” system called Raman Tweezers Microscopy. This analytical spectroscopic technique combines optical trapping with Raman probing. The main idea consists in utilizing the same laser radiation for optical trapping of single nano-bioparticle of interest in aqueous medium and excitation of Raman scattering from the particle’s constituent biomolecules. We are mainly interested in extracellular vesicles, although other bioparticles like lipoproteins, viruses and bacteriophages, liposomes, etc. can also be studied. All major biomolecules (proteins, lipids, nucleic acids, carotenoids, etc.) can be analysed both on the surface and within the bioparticle’s volume providing global biomolecular content characterization. RTM technique does not require external labeling. It can be used for EVs quality-control assessment, and for many other analytical tasks, via the quantitative analysis of EVs biomolecular composition at the single-particle level in physiological conditions.
- Machine Learning: we are developing machine learning-based processes designed to enhance the analysis of multimodal extracellular vesicle (EV) characterization data obtained from different equipment/detectors available at NABI lab and especially within the IVETh expertise facility. This multimodal approach enables us to capture the whole complexity of EV features with the goal of designing new diagnostic workflows (to differentiate EVs from patients presenting a disease from healthy people), as well as for bioproduction quality control tasks to distinguish compliant EV batches from non-compliant ones.
These technologies are integral to the IVETH facility, which is described in detail on a separate page.
By integrating these advanced tools with a strong foundation in physics, our team contributes to groundbreaking discoveries at the crossroads of physical science, biology, and medicine.
Our most recent papers (since 2020)
Sánchez, Sofía V; Otavalo, Gabriela N; Gazeau, Florence; Silva, Amanda KA; Morales, Javier O; Intranasal delivery of extracellular vesicles: A promising new approach for treating neurological and respiratory disorders, Journal of Controlled Release, 379,489-523,2025.
https://doi.org/10.1016/j.jconrel.2025.01.018
https://www.sciencedirect.com/science/article/abs/pii/S0168365925000203
Andrade, Andre Cronemberger; Le Goas, Oriane; Lemieux, Shony; Grangier, Alice; Nicolai, Alice; Guerrera, Chiara; Ribes, Christopher; Suply, Estelle; Volatron, Jeanne; Gazeau, Florence; Silva, Amanda KA; Piezo1 activation increases the release of therapeutic extracellular vesicles after mechanical stimulation in bioreactors,bioRxiv,2025.01. 09.632205,2025
https://doi.org/10.1101/2025.01.09.632205
https://www.biorxiv.org/content/10.1101/2025.01.09.632205v1
Alexandre, Lucile; Arrigo, Daniele D; Kuszla, Nicolas; Andrade, Andre Cronemberger; Silva, Amanda KA; Schietroma, Cataldo; Gazeau, Florence; Lubart, Quentin; Mangenot, Stephanie; Illuminating extracellular vesicles biology with super resolution microscopy: Insights into morphology and composition, arXiv preprint arXiv:2501.03713,2025.
https://doi.org/10.48550/arXiv.2501.03713
https://arxiv.org/abs/2501.03713
Alric, Hadrien; Mathieu, Noëlle; Sebbagh, Anna; Peré, Guillaume; Demarquay, Christelle; Cronemberger, André; Berger, Arthur; Marcel, Benjamin; Wilhelm, Claire; Gazeau, Florence; et al. Thermoresponsive gel embedding extracellular vesicles from adipose stromal cells improves the healing of colonic anastomoses following irradiation in rats, Communications Biology,7,1,1673,2024
https://doi.org/10.1038/s42003-024-07364-2
https://asnr.hal.science/irsn-04877808v1
Mariani, Antoine; Guichard, Augustin; Sebbagh, Anna C; Cronemberger Andrade, André; Al Amir Dache, Zahra; Ribes, Christopher; Ayollo, Dmitry; Karoui, Mehdi; Lavieu, Gregory; Gazeau, Florence; et al. Therapeutic potential of human mesenchymal stromal cell-derived mitochondria in a rat model of post-surgical digestive fistula: towards an energetic nano-biotherapy,bioRxiv,,,2024.11. 29.626014,2024.
https://doi.org/10.1101/2024.11.29.626014
https://www.biorxiv.org/content/10.1101/2024.11.29.626014v1
Alexandre, Lucile; Dubrova, Anastasiia; Kunduru, Aruna; Surply, Estelle; Ribes, Christopher; Boucenna, Imane; Gazeau, Florence; Silva, Amanda KA; Mangenot, Stéphanie; Aubertin, Kelly; Investigating Extracellular Vesicles in Viscous Formulations: Interplay of Nanoparticle Tracking and Nanorheology via Interferometric Light Microscopy, Small Science, 5,1,2400319,2025.
https://doi.org/10.1002/smsc.202400319
https://cnrs.hal.science/MSC-LAB/hal-04795397v1
Thouvenot, Elliot; Charnay, Laura; Burshtein, Noa; Guigner, Jean‐Michel; Dec, Léonie; Loew, Damarys; Silva, Amanda KA; Lindner, Anke; Wilhelm, Claire; High‐Yield Bioproduction of Extracellular Vesicles from Stem Cell Spheroids via Millifluidic Vortex Transport, Advanced Materials,2412498,2024
https://doi.org/10.1002/adma.202412498
https://pubmed.ncbi.nlm.nih.gov/39530646/
Chauvin B., Nakazawa K., Mangenot S. and Bertin A., Reconstituted in vitro systems to reveal the role and functions of septines, Journal of cell Science, 136 (19) (2023)
Ortiz Peña, Nathaly; Cherukula, Kondareddy; Even, Benjamin; Ji, Ding‐Kun; Razafindrakoto, Sarah; Peng, Shiyuan; Silva, Amanda KA; Ménard‐Moyon, Cécilia; Hillaireau, Hervé; Bianco, Alberto; Resolution of MoS2 nanosheets‐induced pulmonary inflammation driven by nanoscale intracellular transformation and extracellular‐vesicle shuttles, Advanced Materials, 35,13,2209615,2023
https://doi.org/10.1002/adma.202209615
https://pubmed.ncbi.nlm.nih.gov/36649533/
Nakazawa K., Kumar G., Chauvin B., Di Cicco A., Pellegrino L., Trichet M., Hajj B., Cabral J., Sain A., Mangenot S. #, Bertin A. #, A human septin octamer complex sensitive to membrane curvature drives membrane deformation with a specific mesh-like organization, Journal of Cell Science 136 (11) (2023)
Piffoux, Max; Silva, Amanda Karine Andriola; Gazeau, Florence; Tareste, David; Generation of hybrid extracellular vesicles by fusion with functionalized liposomes,Membrane Trafficking: Methods and Protocols,385-396,2022, Springer
https://doi.org/10.1007/978-1-0716-2209-4_24
https://pubmed.ncbi.nlm.nih.gov/35819777/
Silva, Amanda; Sagné, Corinne; Gazeau, Florence; Abasolo, Ibane; ,Enzyme replacement therapy: Current challenges and drug delivery prospects via extracellular vesicles,Rare Disease and Orphan Drugs Journal,1,3,13,2022
http://dx.doi.org/10.20517/rdodj.2022.09
https://shs.hal.science/halshs-03867211/document
Alric, Hadrien; Caudron, Eric; Berger, Arthur; Daupin, Johanne; Perrod, Guillaume; Wilhelm, Claire; Gazeau, Florence; Silva, Amanda KA; Rahmi, Gabriel; Anastomotic leak after colorectal surgery: Management by combined use of an over-the-scope-clip and a thermoresponsive gel, Clinics and Research in Hepatology and Gastroenterology,46,9,101990,2022
https://doi.org/10.1016/j.clinre.2022.101990
https://www.sciencedirect.com/science/article/abs/pii/S2210740122001243?via%3Dihub
Piffoux, Max; Silva, Amanda KA; Gazeau, Florence; Salmon, Hugo; Potential of on‐chip analysis and engineering techniques for extracellular vesicle bioproduction for therapeutics, View, 3,1,20200175, 2022
https://doi.org/10.1002/VIW.20200175
https://onlinelibrary.wiley.com/doi/full/10.1002/VIW.20200175
Sailliet, Nicolas; Ullah, Matti; Dupuy, Amandine; Silva, Amanda KA; Gazeau, Florence; Le Mai, Hoa; Brouard, Sophie; Extracellular vesicles in transplantation, Frontiers in immunology,13,800018,2022
https://doi.org/10.3389/fimmu.2022.800018
https://pmc.ncbi.nlm.nih.gov/articles/PMC8851566/
Chauvin B., Nakazawa K., Beber A., Di Cicco A., Hajj B., Iv F., Mavrakis M., Koenderink G., Cabral J.T., TrichetM. , Mangenot S. #, Bertin A. #, Bottom-Up In Vitro Methods to Assay the Ultrastructural Organization, Membrane Reshaping, and Curvature Sensitivity Behavior of Septins, Journal of Vizualized Experiments: JoVE 186 (2022)
Aubertin, Kelly; Piffoux, Max; Sebbagh, Anna; Gauthier, Jeanne; Silva, Amanda KA; Gazeau, Florence; Therapeutic applications of extracellular vesicles, Medecine Sciences: M/S,37,12,1146-1157,2021
https://doi.org/10.1051/medsci/2021207
https://www.medecinesciences.org/en/articles/medsci/full_html/2021/12/msc200672/msc200672.html
Silva, Amanda KA; Morille, Marie; Piffoux, Max; Arumugam, Surendar; Mauduit, Phlippe; Larghero, Jérôme; Bianchi, Arnaud; Aubertin, Kelly; Blanc-Brude, Olivier; Noël, Danièle; et al. Development of extracellular vesicle-based medicinal products: A position paper of the group “Extracellular Vesicle translatiOn to clinicaL perspectiVEs–EVOLVE France”, Advanced Drug Delivery Reviews,179,114001,2021
https://doi.org/10.1016/j.addr.2021.114001
https://pubmed.ncbi.nlm.nih.gov/34673131/
Piffoux, Max; Volatron, Jeanne; Silva, Amanda KA; Gazeau, Florence; Thinking quantitatively of RNA-based information transfer via extracellular vesicles: lessons to learn for the design of RNA-loaded EVs, Pharmaceutics,13,11,1931,2021
https://doi.org/10.3390/pharmaceutics13111931
https://dumas.ccsd.cnrs.fr/STORM/hal-03453201v1
van Niel, Guillaume; Gazeau, Florence; Wilhelm, Claire; Silva, Amanda KA; Technological and translational challenges for extracellular vesicle in therapy and diagnosis, Advanced Drug Delivery Reviews, 114026,2021
https://doi.org/10.1016/j.addr.2021.114026
https://inserm.hal.science/inserm-03421395/document
Piffoux, Max; Volatron, Jeanne; Cherukula, Kondareddy; Aubertin, Kelly; Wilhelm, Claire; Silva, Amanda KA; Gazeau, Florence; Engineering and loading therapeutic extracellular vesicles for clinical translation: A data reporting frame for comparability, Advanced Drug Delivery Reviews, 178, 113972,2021
https://doi.org/10.1016/j.addr.2021.113972
https://hal.science/hal-03453190v1
Coffin, Elise; Grangier, Alice; Perrod, Guillaume; Piffoux, Max; Marangon, Iris; Boucenna, Imane; Berger, Arthur; M’Harzi, Leila; Assouline, Jessica; Lecomte, Thierry; Extracellular vesicles from adipose stromal cells combined with a thermoresponsive hydrogel prevent esophageal stricture after extensive endoscopic submucosal dissection in a porcine model, Nanoscale, 13,35,14866-14878,2021
https://doi.org/10.1039/D1NR01240A
http://pubs.rsc.org/en/content/articlelanding/2021/nr/d1nr01240a
Sebbagh, Anna C; Rosenbaum, Boris; Péré, Guillaume; Alric, Hadrien; Berger, Arthur; Wilhelm, Claire; Gazeau, Florence; Mathieu, Noëlle; Rahmi, Gabriel; Silva, Amanda KA; Regenerative medicine for digestive fistulae therapy: benefits, challenges and promises of stem/stromal cells and emergent perspectives via their extracellular vesicles”, Advanced Drug Delivery Reviews, 179,,113841,2021
https://doi.org/10.1016/j.addr.2021.113841
https://www.sciencedirect.com/science/article/abs/pii/S0169409X21002337
Grangier, Alice; Branchu, Julien; Volatron, Jeanne; Piffoux, Max; Gazeau, Florence; Wilhelm, Claire; Silva, Amanda KA; Technological advances towards extracellular vesicles mass production, Advanced Drug Delivery Reviews,176,113843,2021
https://doi.org/10.1016/j.addr.2021.113843
https://hal.science/hal-03452584
Pinto, Amandine; Marangon, Iris; Méreaux, Julie; Nicolás-Boluda, Alba; Lavieu, Grégory; Wilhelm, Claire; Sarda-Mantel, Laure; Silva, Amanda KA; Pocard, Marc; Gazeau, Florence; Immune reprogramming precision photodynamic therapy of peritoneal metastasis by scalable stem-cell-derived extracellular vesicles, Acs Nano,15,2,3251-3263,2021
https://doi.org/10.1021/acsnano.0c09938
https://pubs.acs.org/doi/10.1021/acsnano.0c09938
Vial A., Taveneau C., Costa L., Chauvin B., Nasrallah H., Godefroy C., Dosset P., Isambert H., Ngo K.X, Mangenot S., Levy D., Bertin A., Milhiet P.E., Correlative AFM and fluorescence imaging demonstrate nanoscale membrane remodeling and ring-like and tubular structure formation by septins, Nanoscale 13, 12484-12493 (2021).
Bhatt, Shvetank; Kanoujia, Jovita; Dhar, Arghya K; Arumugam, Surendar; Silva, Amanda KA; Mishra, Neeraj; Exosomes: a novel therapeutic paradigm for the treatment of depression, Current drug targets,22,2,183-191,2021
http://dx.doi.org/10.2174/1389450121999201006193005
https://hal.science/hal-03097048/document
Alqabandi M., de Franceschi N., Maity S., Miguet N., Roos W., Weissenhorn W., Bassereau P., Mangenot S., The ESCRT-III isoforms CHMP2A and CHMP2B display different effects on membranes upon polymerization, BMC Biology 19, 66 (2021).
DOI : 10.1186/s12915-021-00983-9
Berger, Arthur; Araújo-Filho, Irami; Piffoux, Max; Nicolás-Boluda, Alba; Grangier, Alice; Boucenna, Imane; Real, Caroline Cristiano; Marques, Fabio Luiz Navarro; de Paula Faria, Daniele; Do Rego, Amália Cinthia Meneses; Local administration of stem cell-derived extracellular vesicles in a thermoresponsive hydrogel promotes a pro-healing effect in a rat model of colo-cutaneous post-surgical fistula, Nanoscale,13,1,218-232,2021
https://doi.org/10.1039/D0NR07349K
https://pubs.rsc.org/en/content/articlelanding/2021/nr/d0nr07349k
Millard, Marie; Posty, Solène; Piffoux, Max; Jasniewski, Jordane; Lassalle, Henri-Pierre; Yakavets, Ilya; Gazeau, Florence; Wilhelm, Claire; Silva, Amanda KA; Bezdetnaya, Lina; Mthpc-loaded extracellular vesicles significantly improve mthpc diffusion and photodynamic activity in preclinical models, Pharmaceutics, 12,7,676,2020
Team 3 (emerging): Innovative Cancer Treatments
Team Leader: Laura FOUASSIER, PhD (Rsearcher, INSERM)
Team members:
PUPH: M Pocard, P Soyer, B Malgras
PH: M Barral, R Kaci, A Fazel
C Crocheray – IE UPC
M Meunier—Thaens – IE CDD, M Guettab – AI
Post doc: M Minini, M Janona, S Becharef, G Avveduto
Doc: A Leinebo, E M Biaffeu, C Nkembe
M2: F Nedhif, I Linganathan
Emerites: JP Thiery (CNRS), M Mishashi
Nanomedicine approaches to modulate the microenvironment of solid tumors
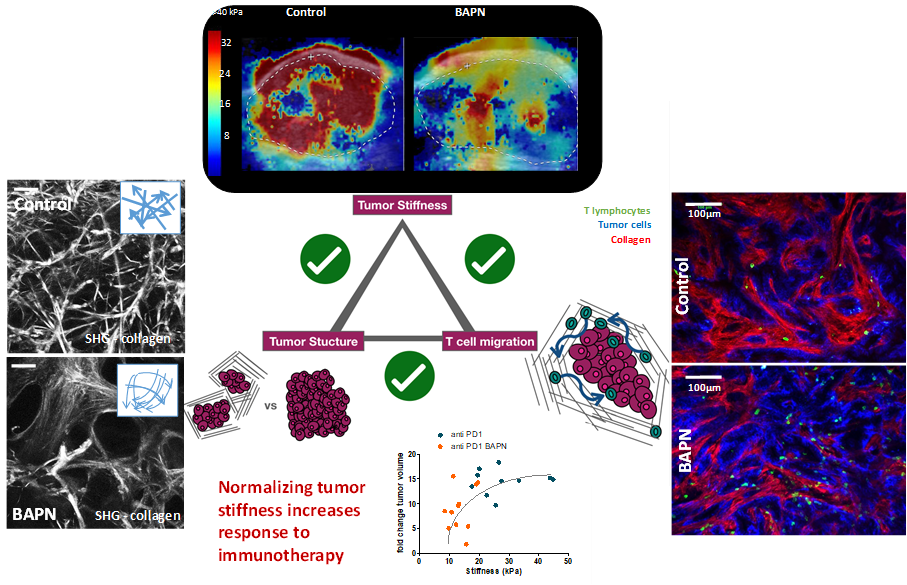
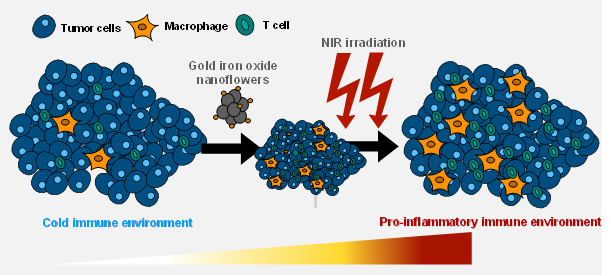
Solid tumors are heterogeneous ever evolving ecosystem comprising not only tumor cells but also a stroma containing fibroblasts, extracellular matrix, immune cells, with aberrant physical properties in comparison to healthy tissue (increased internal pressure, increased stiffness, chaotic vascularisation, hypoxia…). Desmoplastic tumors such as pancreatic ductal adenocarcinoma or cholangiocarcinoma are characterized by a very stiff extracellular matrix which raises a barrier against the penetration of chemotherapy as well as immune cells, which can explain the resistance of such cancer to most current treatment as well as immunotherapeutic approaches. Tackling the tumor microenvironment and normalizing its physical properties is an attractive strategy to make the tumor more sensitive to chemo and immunotherapies. In a recent study, we established the link between tumor architecture, tumor stiffening and the ability of T cell to infiltrate the tumor (elife 2021). We are developping nanomedicine approaches to modulate the tumor microenvironment with a spatial and temporal control owing to the external activation of nanoparticles
How tumor stiffness affect immune cell infiltration?
Solid tumors are heterogeneous ever evolving ecosystem comprising not only tumor cells but also a stroma containing fibroblasts, extracellular matrix, immune cells, with aberrant physical properties in comparison to healthy tissue (increased internal pressure, increased stiffness, chaotic vascularisation, hypoxia…). Desmoplastic tumors such as pancreatic ductal adenocarcinoma or cholangiocarcinoma are characterized by a very stiff extracellular matrix which raises a barrier against the penetration of chemotherapy as well as immune cells, which can explain the resistance of such cancer to most current treatment as well as immunotherapeutic approaches. Tackling the tumor microenvironment and normalizing its physical properties is an attractive strategy to make the tumor more sensitive to chemo and immunotherapies. In a recent study, we established the link between tumor architecture, tumor stiffening and the ability of T cell to infiltrate the tumor (elife 2021). We are developping nanomedicine approaches to modulate the tumor microenvironment with a spatial and temporal control owing to the external activation of nanoparticles.
Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. Alba Nicolas-Boluda, Javier Vaquero, Lene Vimeux, Thomas Guilbert, Sarah Barrin, Chahrazade Kantari-Mimoun, Matteo Ponzo, Gilles Renault, Piotr Deptula, Katarzyna Pogoda, Robert Bucki, Ilaria Cascone, José Courty, Laura Fouassier, Florence Gazeau*, Emmanuel Donnadieu*
eLife 2021;10:e58688 doi: 10.7554/eLife.58688
Videos:
Treated with BAPN – https://static-movie-usa.glencoesoftware.com/mp4/10.7554/442/776d6889c8c8397cd70f8490229c2e342d35e7f9/elife-58688-video1.mp4
Nanohyperthermia to reverse stiffness in desmoplastic tumors
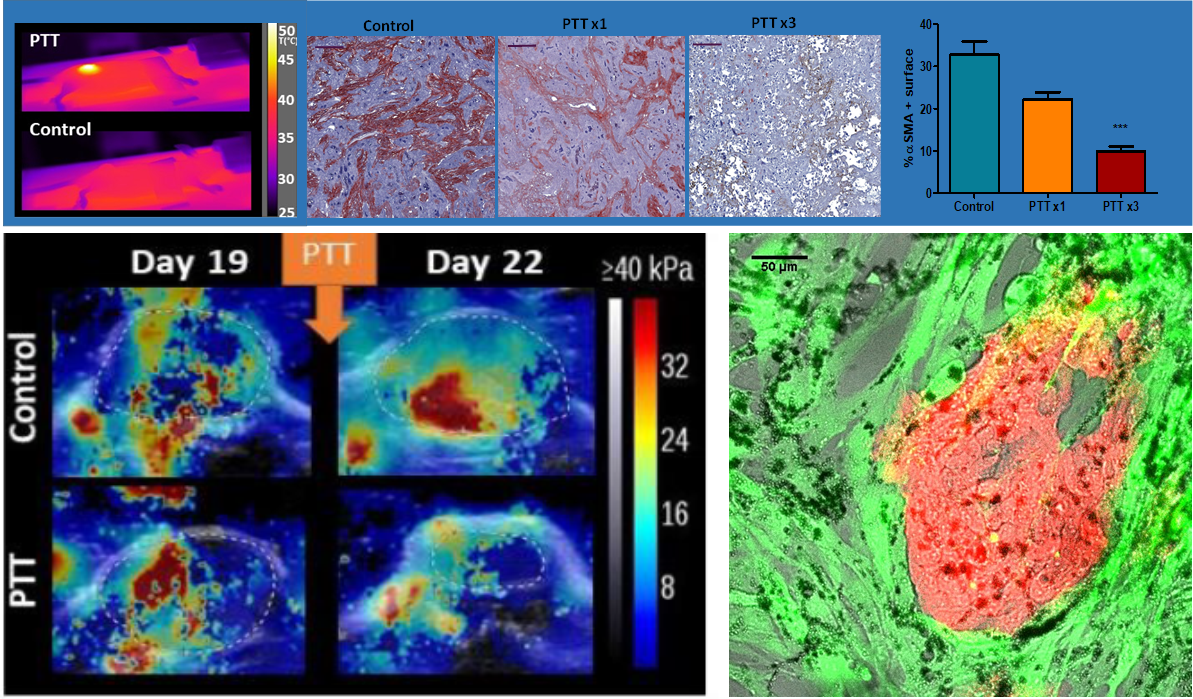
Photothermal Depletion of Cancer-Associated Fibroblasts Normalizes Tumor Stiffness in Desmoplastic Cholangiocarcinoma. A Nicolás-Boluda, J Vaquero, G. Laurent, G Renault, R Bazzi, E Donnadieu, S Roux, L Fouassier,* F Gazeau*. ACS Nano, 2020, 14, 5, 5738–5753 doi.org/10.1021/acsnano.0c00417
Gold decorated iron oxide Nanohyperthermia modulate tumor immune response

Two step promotion of a hot tumor immune environment by gold decorated iron oxide nanoflowers and light-triggered mild hyperthermia, Alba Nicolas-Boluda, Gautier Laurent, Rana Bazzi, Stéphane Roux, Emmanuel Donnadieu and Florence Gazeau, Nanoscale, 2021, 13, 18483–18497 DOI: 10.1039/d1nr03201a
Nanohyperthermia as an alternative to HIPEC (hyperthermic intraperitoneal chemotherpy) to treat peritoneal metastasis
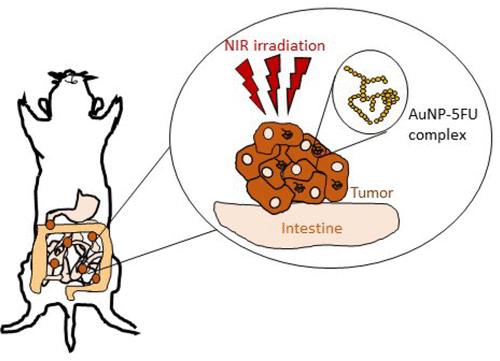
Tumor-Selective Immune Active Mild Hyperthermia Associated with Chemotherapy in Colon Peritoneal Metastasis by Photoactivation of Fluorouracil – Gold Nanoparticles Complexes V Mulens-Arias, A Nicolás-Boluda, A Pinto, A Balfourier, F Carn, A K A Silva, M Pocard, F Gazeau* ACS Nano, February 2, 2021 DOI:10.1021/acsnano.0c10276
Nanohyperthermia : towards intracellular precision
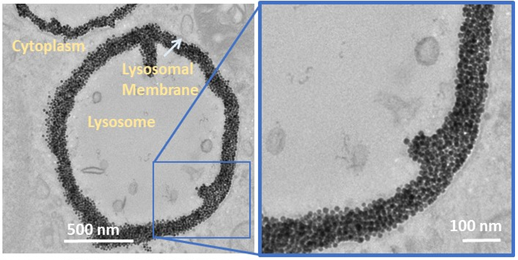
Intracellular Fate of Hydrophobic Nanocrystal Self‐Assemblies in Tumor Cells
A Nicolas‐Boluda, Z Yang, I Dobryden, F Carn, N Winckelmans, C Péchoux, P Bonville, S Bals, P Martin Claesson, F Gazeau*, M P Pileni * Advanced Functional Materials,16 August 2020 https://doi.org/10.1002/adfm.202004274
Self-assemblies of Fe3O4 nanocrystals: towards nanoscale precision of photothermal effects in the tumor microenvironment. A Nicolas-Boluda, Z Yang, T Guilbert, L Fouassier, F Carn, F Gazeau,* MP Pileni*
Advanced functional materials 2020/10/15 2006824 https://doi.org/10.1002/adfm.202006824
Immune Reprogramming Precision Photodynamic Therapy of Peritoneal Metastasis by Scalable Stem Cell Derived Extracellular Vesicles
Immune Reprogramming Precision Photodynamic Therapy of Peritoneal Metastasis by Scalable Stem Cell Derived Extracellular Vesicles” A Pinto, I Marangon, J Méreaux, A Nicolás-Boluda, G Lavieu, C Wilhelm, L Sarda, A K A Silva, M Pocard*, F Gazeau* ACS Nano, January 22, 2021, https://doi.org/10.1021/acsnano.0c09938
Team IVETh – national biotherapy-bioproduction integrator dedicated to extracellular vesicles
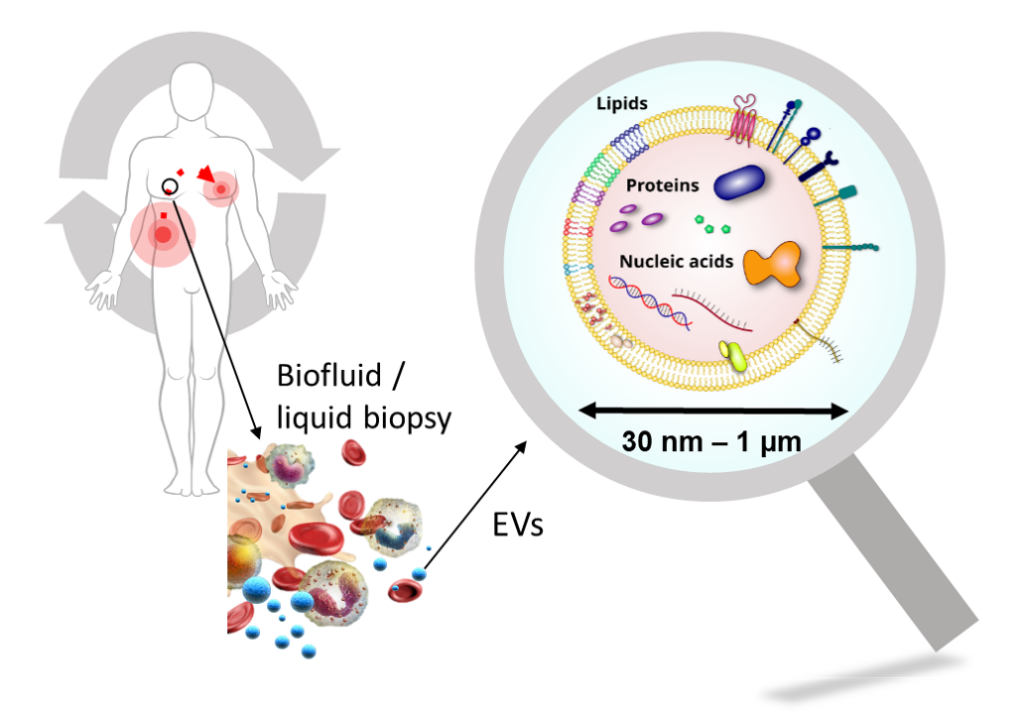
Extracellular vesicles (EVs) are submicrometric (30 – 1000 nm) biological objects delimited by a membrane and secreted by most of cell types. They are central to the intercellular communication and the process of molecular exchange between intra- and extracellular media. By recapitulating the biological properties of producer cells, EVs have a increasing interest in translationnal research, especially for theranostics, ie therapeutic and / or diagnostic applications.
Indeed, in a physiopathological context, EVs are natural diagnostic probes circulating in lots of biological fluids (blood, saliva, urine, …) and could be accessible through minimally invasive methods.
When secreted by stem cells, EVs displayed regenerative properties similar to producer cells, offering therefore an alternative solution to cell therapy for regenerative medicine. Their biocompatibility is also a great advantage for their use as therapeutic vectors for drug delivery (ex: chemotherapy) with in addition a preferential organostropism for inflammation sites.
In order to design an analytical toolbox for the quality control of therpeutic EVs as well as to design diagnostic tools based on EVs from different biofluids, MSC-Med created a Core Facility called IVETh for the production, engineering and characterization of EVs for diagnosis and personalized therapy.
Physicists, biologists, chemists, pharmacists and clinicians gathered together within the IVETh core facility to offer a panel of expertises around the physical, chemical and biological characterization of EVs for therapeutic or diagnostic purposes. The IVETh core facility regroups state-of-the-art equipments that are distributed over 4 sites (MSC-Med, IPNP, CRI, PARCC).

Our facility proposed a panel of cutting edge instruments for characterizing EV size and concentration (based on NTA, ILM), EV biomolecular signature (based on Raman spectroscopy), EV phenotype (based on interferometric detection on immunofunctionnalized substrates), therapeutical potency (based on high content screening & analysis). In addition, the facility also offers access to analytical separation (A4F), purification (UC, TFF) and nanoscopy.

We are also developing statistical methodologies based on machine learning to develop automated classification based on multimodal data.
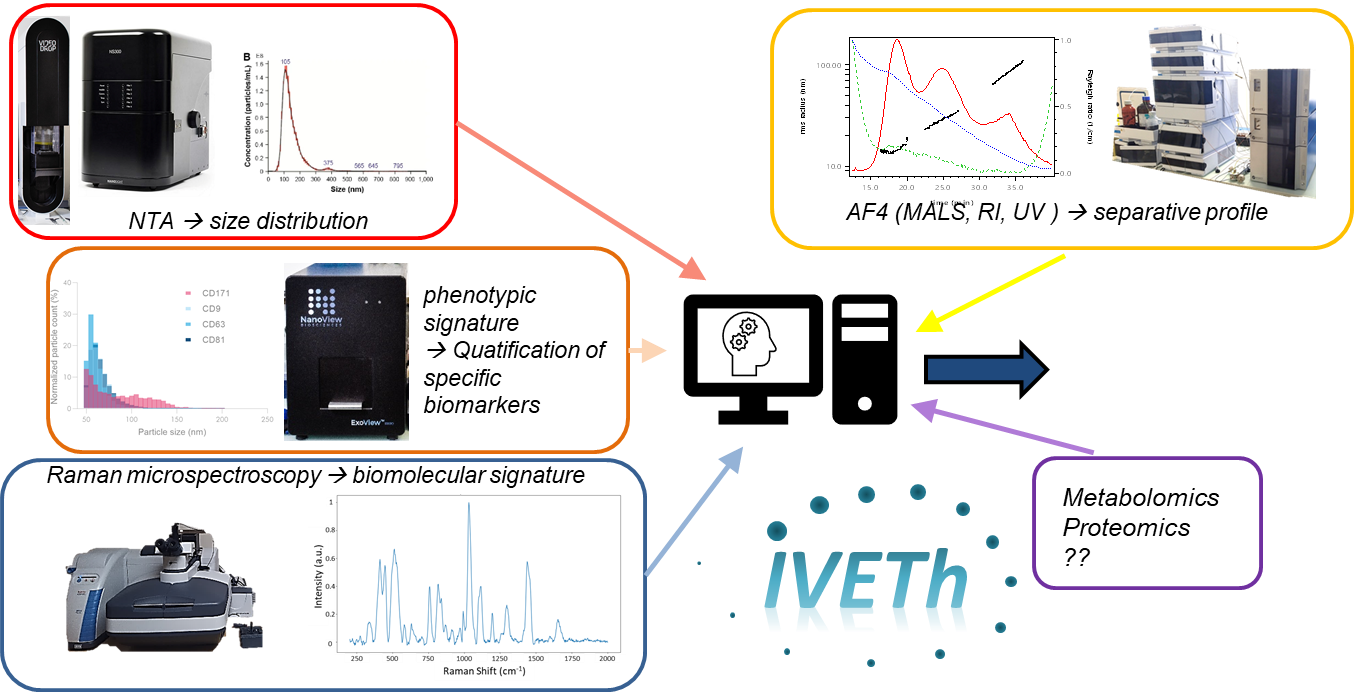
For more information about our IVETh Core Facility, please visit the dedicated website here.
Team ATIP Avenir: Mucosal Viral Immune Responses (MuVIRep)
Team leader: Nikaïa Smith (CRCN, INSERM)
Members: Dominique Cathelin, PhD (Engineer), Maxime Neto (Master student 2), Juliette Guyard (Master student 2)
Research Topic I: Effect of polyamines in the regulation of immune responses
HIV-1 is primarily transmitted through sexual contact, yet the mechanisms by which the virus overcomes mucosal immune defenses remain poorly understood. Our lab investigates the role of semen-derived polyamines (spermine and spermidine) in modulating innate immunity and facilitating HIV-1 transmission. Our recent findings indicate that these polyamines bind to CXCR4, a key HIV-1 coreceptor, suppressing type I interferon (IFN-I) responses in plasmacytoid dendritic cells (pDCs) and inducing a senescence-like state. This immune suppression may create a permissive environment at mucosal surfaces, favoring viral replication and transmission. Additionally, our data suggest that spermine and spermidine selectively inhibit X4-tropic HIV-1 infection while having no effect on R5-tropic strains, potentially explaining why sexually transmitted founder viruses R5-tropic are almost exclusively. However, the broader implications of polyamine-induced immune modulation in mucosal tissues remain unexplored.
Our study aims to provide fundamental insights into how semen modulates mucosal immunity, thereby contributing to immune evasion and HIV-1 persistence. By identifying the key molecular players involved in polyamine-induced immune suppression, we seek to develop novel therapeutic strategies to counteract these effects. Specifically, targeting polyamine metabolism or blocking polyamine-CXCR4 interactions could help restore innate immune function at mucosal surfaces. In this context, polyamine inhibitors could be repurposed to neutralize polyamine-induced immune suppression and reinforce mucosal antiviral defenses.
The expected outcomes of this project include (1) a detailed molecular understanding of semen-induced immune modulation, (2) validation of polyamines as immunosuppressive mediators in HIV-1 transmission, and (3) the identification of potential intervention strategies to restore immune defenses. Given the broad role of polyamines in immune regulation, our results may also have implications for other sexually transmitted infections (STIs), chronic inflammatory diseases, and cancer immunology. By bridging the gap between fundamental immunology and translational medicine, this project aims to contribute to novel approaches for HIV prevention and immune system restoration.
Research Topic II: Unraveling the Role of Polyamines in Male Fertility and Female Mucosal Immunity
Male infertility affects approximately 7% of men of reproductive age, with multiple contributing factors, including metabolic and immune system interactions. Polyamines (putrescine, spermidine, spermine), found in high concentrations in semen, are essential for cell cycle regulation, DNA stability, and oxidative stress management. Emerging evidence suggests that disruptions in polyamine metabolism contribute to sperm abnormalities and reduced fertilization capacity. Additionally, polyamines possess immunomodulatory properties that may influence the female immune environment, impacting sperm tolerance and embryo implantation. However, the interactions between semen polyamines and the female mucosal immune response remain poorly understood.
Our team aims to investigate the role of polyamines in male infertility and their impact on the vaginal mucosal immune system. Specifically, we will explore (1) correlations between polyamine levels, sperm quality, and male fertility, and (2) how sperm-derived polyamines influence immune responses in the female reproductive tract. To overcome current limitations in polyamine quantification, we propose an innovative approach using functionalized gold nanoclusters (AuNCs) with unique optical and electrochemical properties. This cost-effective, rapid detection method will improve polyamine measurement in sperm samples, allowing for better clinical diagnostics in infertility.
The project integrates analytical chemistry, reproductive medicine, and immunology, leveraging cutting-edge techniques such as flow cytometry, RT-qPCR, and high-sensitivity digital ELISA to assess immune responses. Using semen samples from men with idiopathic infertility and fertile controls, we will correlate polyamine levels with sperm motility, DNA integrity, and oxidative stress markers. Additionally, transcriptomic analyses and ex vivo cervicovaginal tissue models will be used to evaluate the effects of semen polyamines on immune cell activation, phagocytosis, and mucosal barrier integrity.
By elucidating the immuno-metabolic interactions between polyamines, sperm function, and the female reproductive tract, this research will provide novel insights into fertility mechanisms and potential biomarkers or therapeutic targets for male infertility.
Selected Relevant Publications:
Full list : https://orcid.org/0000-0002-0202-612X
- Bekaddour N, Smith N, Caspar B, Grinberg S, Giorgiutti S, Rodeschini V, Dupuy S, Leboulanger N, Duffy D, Soulas-Sprauel P, Gies V, Korganow AS, Nisole S, Herbeuval JP. The histamine analogue clobenpropit modulates IRF7 phosphorylation and interferon production by targeting CXCR4 in systemic lupus erythematosus models. Front Immunol. 2024 Dec 16;15:1490593. doi: 3389/fimmu.2024.1490593
- Bekaddour N, Smith N, Beitz B, Llibre A, Dott T, Baudry A, Korganow AS, Nisole S, Mouy R, Breton S, Bader-Meunier B, Duffy D,Terrier B, Schneider B, Quartier P, Rodero MP, Herbeuval JP. Targeting the chemokine receptor CXCR4 with histamine analogue to reduce inflammation in juvenile arthritis: a proof of concept for COVID-19 therapeutic approach. Frontiers in Immunology. 2023 Sep 26;14:1178172. doi:3389/fimmu.2023.1178172
- Harms M*, Smith N*, Han M, Groß R, von Maltitz P, Ruiz-Blanco Y.B, Almeida-Hernández Y, Rodriguez-Alfonso A, Sayettat S, Cathelin D, Bekaddour N, Caspar B, Tahar B, Vanshylla K, Kleipass F, Wiese S, Ständker L, Klein F, Lagane B, Boonen A, Schols D, Benichou S, Sanchez-Garcia E, Herbeuval JP*, Münch*. Spermine and spermidine bind CXCR4 and inhibit CXCR4- but not CCR5-tropic HIV-1 infection. Science Advances. 2023 July 5;9,eadf825. doi:1126/sciadv.adf82512023
- Matuozzo D,…, Casanova JL, Zhang Q, Abel L, Cobat A. Rare predicted loss-of-function variants of type I IFN immunity genes are associated with life-threatening COVID-19. Genome Med. 2023 Apr 5;15(1):22. doi: 1186/s13073-023-01173-8
- Smith N*, Possémé C*, Bondet V*, Sugrue J, Townsend L, Charbit B, Rouilly V, Saint-André V, Dott T, Rodriguez Pozo A, Yatim N, Schwartz O, Cervantes-Gonzales M, Ghosn J, Bastard P, Casanova JL, Szwebel TA, Terrier B, Conlon N, O’Farrelly C, Ní Cheallaigh C, Bourke NM, Duffy D. Defective type I interferon immunity is associated with increasing COVID-19 severity. Nature Communications. 2022 Nov 25;13(1):7254. doi: 1038/s41467-022-34895-1
- Smith N*, Goncalves P*, Charbit B, Grzelak L, Beretta M, Planchais C, Bruel T, Rouilly V, Bondet V, Hadjadj J, Yatim N, Pere H, Merkling SH, Ghozlane A, Kernéis S, Rieux-Laucat F, Terrier B, Schwartz O, Mouquet H, Duffy D, Di Santo J§. Distinct systemic and mucosal immune responses to SARS-CoV-2. Nature Immunology. 2021 Nov;22(11):1428-1439. DOI:10.1038/s41590-021-01028-7
- Hadjadj J*, Yatim N*, Barnabei L, Corneau A, Boussier J,Smith N, Péré H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pène F, Marin N, Roche N, Szwebel TA, Merkling SH, Treluyer JM, Veyer D, Mouthon L, Blanc C, Tharaux PL, Rozenberg F, Fischer A, Duffy D, Rieux-Laucat F, Kernéis S, Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020 Oct 23;370(6515):eabd4570. DOI: 1126/science.abc6027
- Smith N, Bekaddour N, Leboulanger N, Richard Y*, Herbeuval JP*. Isolation of Tonsillar Mononuclear Cells to Study Ex Vivo Innate Immune Responses in a Human Mucosal Lymphoid Tissue. Journal of Visual Experimentation. 2020 Jun 14;(160). DOI: 3791/60914
- Smith N, Rodero MP, Bekaddour N, Bondet V, Ruiz Blanco YB, Harms M, Mayer B, Badder-Meunier B, Quartier P, Bodemer C, Baudouin V, Dieudonnée Y, Kirchhoff F, Sanchez-Garcia E, Charbit B, Leboulanger N, Jahrsdörfer B, Richard Y, Korganow AS, Münch J, Nisole S, Duffy D, Herbeuval JP. Control of TLR7-mediated type I IFN signaling in pDCs through CXCR4 engagement – a new target for Lupus treatment. Science Advances. 2019 Jul 10;5(7):eaav9019. DOI: 1126/sciadv.aav9019
- Smith N, Pietrancosta N, Dutrieux J, Davidson S, Chauveau L, Cutolo P, Dy M, Scott-Algara D, Manoury B, Zirafi O, McCort-Tranchepain I, Durroux T, Bachelerie F, Schwartz O, Münch J, Wack A, Nisole S, Herbeuval JP. Natural amines inhibit activation of human plasmacytoid dendritic cells through CXCR4 engagement. Nature Communications. 2017 Feb 9;8:14253. DOI: 1038/ncomms14253
- Smith N, Vidalain PO, Nisole S, Herbeuval JP. An efficient method for gene silencing in human primary plasmacytoid dendritic cells. Scientific Reports. 2016 Jul 14;6:29891. DOI: 1038/srep29891